
Thus, metallic character increases as we move down a group and decreases across a period in the same trend observed for atomic size because it is easier to remove an electron that is farther away from the nucleus. Another definition that can be found: Electron affinity is the energy released when an electron is added to the valence shell of a gas-phase atom. The electron affinity, EA, of a molecule is, for negative ions or anions, the quantity that is analogous to the ionization energy for positive ions. Metallic properties including conductivity and malleability (the ability to be formed into sheets) depend on having electrons that can be removed easily.

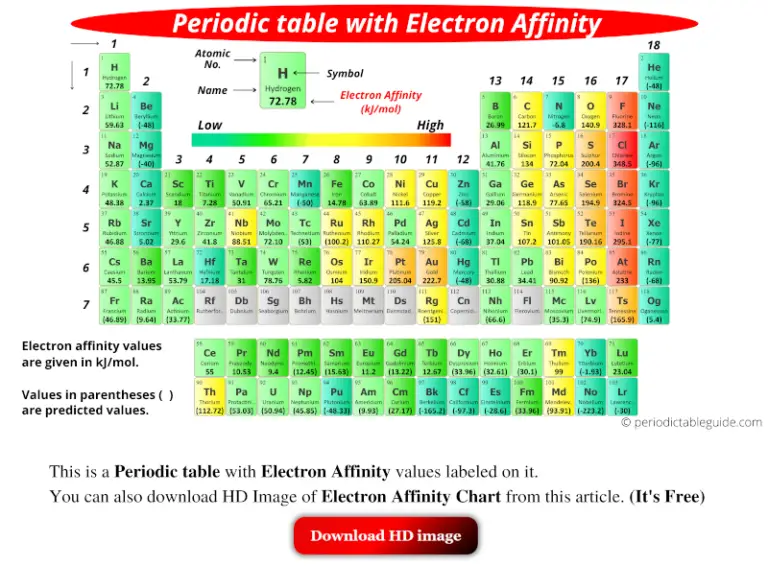
The gray squares represent metals, the orange squares are "metalloids" and the yellow squares are nonmetals.Ĭredit: "Various Periodic Trends" by Sandbh is licensed under CC BY-SA 4.0 \): Metallic character increases as you go from right to left on periods and move down groups on the periodic table. When the value of electron affinity is negative, we have exothermic electron affinity while when the value of electron affinity is positive, we have endothermic. To elucidate the origin of the high electron affinity and stability toward the multi-electron reduction of fullerenes with only a carbon skeleton, we designed and synthesized end-capped 3 and 4.


 0 kommentar(er)
0 kommentar(er)
